The Gut Mucosal Immune System: Where Microbiome Meets Immunity
Food must be ingested, broken down, and absorbed selectively across specialized tissues, allowing rejected items to continue through the gastrointestinal (GI) system and out of the body. Absorption of food is a complicated affair because food contains necessary nutrients along with numerous antigens capable of inducing an immune response, such as gluten or milk proteins. The body must be able to allow food particles to pass into the interior of the body without mounting an overly-vigorous immune response to any food antigens contained within. And yet, should any disease-causing pathogens attempt to gain entry along with the accepted food particles in the small intestine or farther down in the large intestine with the rejected items, the immune system must be ready to destroy them (1). The gut mucosal immune system exists at this point of food intake to guard the body’s interior.
Gut homeostasis, the stability and integrity of the gut, is paramount to human health. One big player in the integrity of the gut, which has been studied extensively, is the community of microorganisms collectively known as the microbiota (or microbiome if considering the genetic component). It is well known now that complex interactions and feedback loops exist between the microbiome, gut, and brain. Yet the microbiome also has bidirectional interactions with the host immune system through layers of mucous and cells known as the mucosal immune system. This article will cover some of the players in this scenario, focusing on the single sheet of cells that hold the line of defense against the vast array of microorganisms of the microbiome.
The Mucosal Immune System in the Gut
Structurally, the mucosal immune system (MIS) of the GI tract is comprised of multiple layers. Starting from the interior, the layers of the gut MIS are the lumen, mucin layers (mucosa and submucosa), then the layers of muscle, lymph, and circulation that signal entry into the body proper. The lumen is the space where food passes through the tube that comprises the GI tract. The mucosa layer contains a single sheet of intestinal epithelial cells (IECs) and the lamina propria (2).
Bacteria, fungi, and other microorganisms that can be identified by genetic analysis (the microbiome) dwell primarily in the mucin layers but also on the epithelial cells’ surface (lumen side). Physiologically, complex interactions and dependencies exist within and among each layer of the mucosal immune system. For example, though the microbiome dwells in the mucin layers, it regularly interacts with the IECs and the lamina propria.
The lamina propria lies below the layer of IECs, comprised of immune cells and connective tissues. The array of immune cells in the small intestine may include plasma cells, macrophages, and lymphocytes. In the large intestine, the lamina propria has a similar composition but has additional structures called crypts, which create intestinal epithelial stem cells.
The lamina propria also holds the GALT, gut-associated lymphoid tissue. Estimates indicate that the plasma-rich GALT is about 70% of the immune system by weight (3). Peyer’s patches are part of the GALT in the small intestine, where they protrude through the layer of IECs into the lumen, covered by M cells that serve as a conduit from the lumen into the Peyer’s patches. M cells examine the contents of the lumen and may bring food particles or microbes across the layer of IECs.
There are slight differences anatomically and physiologically between the mucosal barriers of the small and large intestines. However, they share the same function – they form where vital communication and exchanges between the microbiome and the immune system occur. Multiple things happen at this border. While the small intestine has a significant function of absorbing nutrients at this border, the large intestine does absorb some items (water, electrolytes, and short-chain fatty acids), and they both defend against pathogens and facilitate bidirectional communication between the microbes of the microbiome and the immune system (4).
The Crux – Intestinal Epithelial Cells (IECs)
The single layer of intestinal epithelial cells (IECs) lies in the middle of this highly active mucosal area, a line of defense the immune system uses to keep pathogens out of the interior. Not only do IECs provide a physical barrier, but they also provide a chemical barrier and a means of communication.
The sheet layer of IECs of the gut is comprised of various cells that differ between the small and large intestines. They begin as epithelial stem cells, differentiating into either absorptive or secretory types. Secretory types include enteroendocrine, Paneth, goblet, and tuft cells. Absorptive types include colonocytes, enterocytes, and microfold cells (5).
Goblet cells create the mucin that makes up the mucin layers. Mucin is composed of complex glycoproteins that line the interior of the GI tract, lying in direct contact with the IEC layer. The mucin layer provides the dwelling place for the bulk of the microbiome, as its gel structure traps nutrients and water, providing an ideal place for bacteria and other microbes.
The layer of IECs is held together by two types of cell junctions composed of protein complexes: tight junctions and adherens junctions. Tight junctions lie between individual epithelial cells and are selectively permeable, allowing digested food particles and other necessities to reach the circulatory system. Tight junctions get a lot of attention because they play a part in “leaky gut,” when they no longer function correctly and allow large (incompletely digested) food particles and microbes from the microbiome to enter the rest of the body. Both the large food particles and the microbial translocation get the attention of the immune system, often creating chronic inflammation that is associated with multiple chronic illnesses such as diabetes, inflammatory bowel disease, and some neurological diseases (6).
Roles of intestinal epithelial cells in gut homeostasis
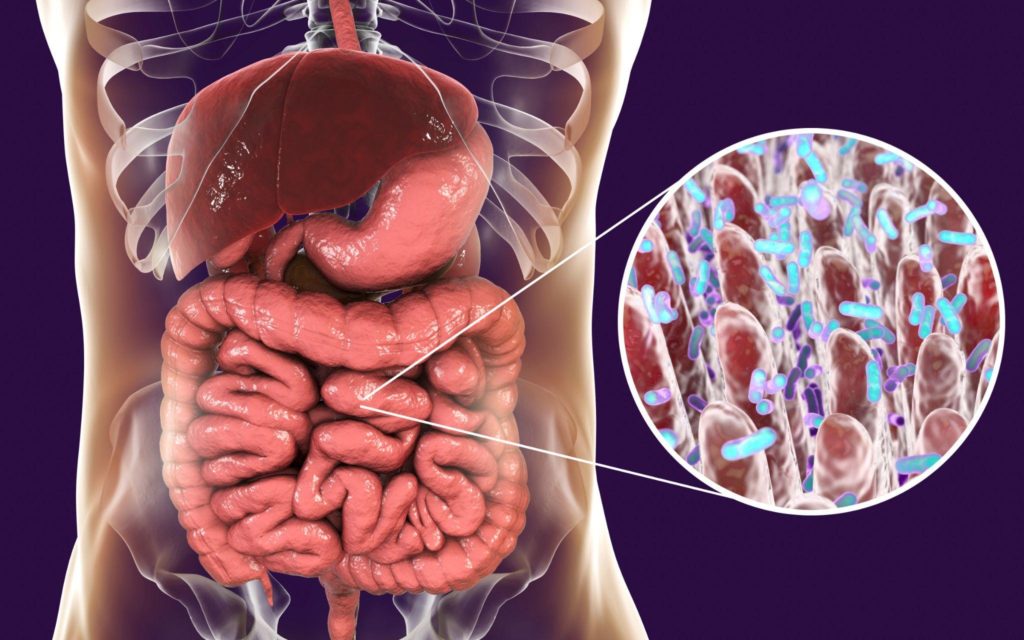
When the interplay between the host, the host’s microbiota, and the host’s immune system is stable, intact, and balanced, the goal of gut homeostasis has been achieved. If the intestinal barrier is breached or the immune response is imbalanced, it would contribute to a dysfunctional system.
In fact, one of the IEC’s leading roles is to keep the microbiome separate from the immune system (7), providing a barrier but also providing a means of cross-talk between the microbiome and the immune system. IECs achieve gut homeostasis through the following means, among others.
1. Defend against pathogens with physical barriers – As described above, IECs form a sheet layer of cells that provide a physical barrier between the inside and outside of the body. Cells in this layer are connected by tight junctions, adherens junctions, and other protein complexes (8). Tight junctions seal the gaps between epithelial cells, identifying and selectively allowing substances into the interior space. Adherens junctions are connections between actin (filaments) in adjacent cells.
Besides the layer of intestinal epithelial cells, another part of the physical barrier is the mucin layer, which is produced by the goblet cells of the IECs and lies directly on the lumen side. The mucin layer keeps the epithelial layer moist, provides a layer of protection by slowing down substances in its gel-like matrix, and provides a place where the gut microbes can live (7).
Additionally, IECs have “glycocalyx,” a layer of glycan molecules that face the lumen side, on the microvilli (9). Glycocalyx assists in the absorption of nutrients and releases enzymes that help break down food and act as a physical barrier (7).
Interestingly, tight junctions can be opened by dendritic cells, allowing them out and into the lumen to kill Salmonella and E. coli bacteria by phagocytosis. The “door” of the tight junctions allows both entry to and exit from the interior space (6).
One of the main functions of IECs is to selectively allow particular substances through their physical barriers. IECs actively guard against potentially harmful items such as toxins, antigens (non-self), and pathogens called pathogen-associated molecular patterns (PAMPs). However, properly functioning tight junctions will allow water, nutrients, and electrolytes to pass into the body’s interior. Typically, what tight junctions allow in is based on size or their charge (8).
Should any junctions be disrupted, the gut can be breached by whatever lies in the lumen- food particles, microbes, or toxins. Disrupted tight junctions contribute to intestinal permeability, which not only allows pathogens into the body proper but can also disrupt the movement of inflammatory mediators, causing inflammation and a potential negative feedback loop of continuing inflammation (8).
2. Defend against pathogens with chemical barriers – In addition to being a physical barrier, IECs produce and maintain numerous chemical barriers. Chemical barriers are another way the body separates the commensal microbes (normal gut flora that is neither harmful nor especially beneficial) from the host’s immune system.
In the small intestine, IECs called Paneth cells create defensin proteins, which are antimicrobial peptides capable of disrupting the cell membranes of various microbes, being particularly efficacious against bacteria. Defensin proteins can poke holes in the membranes of microbes that eventually cause the cell to die.
Another chemical barrier is the mucin layer’s antimicrobial peptides (AMPs), produced by goblet, Paneth, and enterocyte IECs. These can directly kill pathogens, including pathogenic fungi and bacteria. In addition, they can modulate the immune system response by upregulating anti-inflammatory cytokines or downregulating pro-inflammatory cytokines (7). AMPs can also defend gut integrity by increasing the production of mucus for the mucin layer.
3. Provide the means for cross-talk between the gut microbes and the host’s immunity – Diet, medications, and toxins frequently change the makeup of the local environment as these substances are broken down and absorbed in the GI tract. The immune system and the local environment must respond to these changes (7). What facilitates this responsiveness is bidirectional communication, or cross-talk, both direct and indirect.
One case of direct cross-talk between the microbiota and the immune system is lipopolysaccharides in bacterial membranes interacting with immune cells, triggering an immune response. Interestingly, the creation of antimicrobial peptides (AMPs) by Paneth cells is regulated by many signaling pathways, including toll-like receptor 4 (TLR4) and nucleotide-binding oligomerization domain-containing protein 2 (NOD2). Lipopolysaccharides, which are in the cell walls of gram-negative bacteria and are recognized by TLR4, can trigger a cascade of signaling that results in the creation of pro-inflammatory cytokines and chemokines. Toll-like receptors are one of many pattern recognition receptors which epithelial cells express that can be identified and interacted with by microbes. Indeed, gut microbes can actually drive the TLR4 signaling, a way for Paneth cells to be encouraged to produce more antimicrobials (AMPs) (6).
Another case of direct cross-talk between the microbiome and the host immune system is that of short-chain fatty acids (SCFAs). Created by the fermentation of fiber in the gut, SCFAs can modulate innate immunity by increasing the production of AMPs and by interacting with T-regulatory cells to decrease inflammation (10).
Indirectly, microbes can create metabolites that can cross into the body’s interior, travel by the bloodstream, and affect the immune system. One important metabolite created by the microbiota is the neurotransmitter substance serotonin. Serotonin crosses into the circulation, potentially affecting immune cells involved in inflammation called T helper 17 (Th17) cells. Serotonin interacts with receptors on the exterior of the Th17 cells, inhibiting how it differentiates and functions (11).
Segmented filamentous bacteria (SFB) also affect Th17 responses, though the exact mechanism is unknown. SFB can increase Th17 in the gut, which can help protect the host against pathogens. However, this increase can also lead to problems with autoimmunity. Indeed, SFBs are associated with inflammation in the intestines and the triggering of arthritis (6). It is speculated that
SFB presents antigens to pattern recognition receptors, such as TLRs expressed on the surface of innate immune cells.
4. Produce cytokines & chemokines – IECs can produce cytokines and chemokines in response to gut microbes or metabolites. Both cytokines and chemokines are chemical mediators. Cytokines are proteins that have roles in the immune system, especially inflammation. They can inhibit or activate immune responses. Chemokines are a subtype of cytokines that activate immune cells and regulate their migration. These chemical mediators can influence T-cell immune responses, which help prevent infections.
Certain types of stimulation can cause innate lymphoid cells (ILCs) in epithelial cells to produce cytokines to induce or subdue inflammation, which can either protect the host or cause an issue if the inflammation becomes chronic. These ILCs can produce IL-22, which can help heal infections in the gut and induce IECs to create substances that will kill gram-positive bacteria (6).
Cytokines and chemokines can also help educate the immune system by delivering antigens to antigen-presenting cells (APCs) to create antibodies. This process is called the IgA response and has been identified as helping the body to develop oral tolerance to food antigens.
Conclusion:
Not only are the sheet of intestinal epithelial cells a line of demarcation between the interior and the exterior of the body, but they are also the crux of direct and indirect communication between the millions of microbes in the gut and the immune system. IECs are very active in mucosal immunity and play critical roles in defending against pathogens, creating antimicrobial molecules, producing chemical mediators, and even helping to educate the immune system. Nurturing and maintaining this line of defense ensures the health of the host.
Sources:
- Janeway CA Jr, Travers P, Walport M, et al. Immunobiology: The Immune System in Health and Disease. 5th edition. New York: Garland Science; 2001. The mucosal immune system. Available from: https://www.ncbi.nlm.nih.gov/books/NBK27169/
- Vanner SJ, Greenwood-Van Meerveld B, Mawe GM, Shea-Donohue T, Verdu EF, Wood J, et al. Fundamentals of Neurogastroenterology: Basic Science. Gastroenterology. 2016;150(6):1280–91.
- Dr.Samanthi. What is the difference between Balt Galt and malt [Internet]. Compare the Difference Between Similar Terms. Differencebetween.com; 2022 [cited 2023Feb28]. Available from: https://www.differencebetween.com/what-is-the-difference-between-balt-galt-and-malt/
- Fasano A. All disease begins in the (leaky) gut: role of zonulin-mediated gut permeability in the pathogenesis of some chronic inflammatory diseases. F1000Res. 2020 Jan 31;9:F1000 Faculty Rev-69. doi: 10.12688/f1000research.20510.1. PMID: 32051759; PMCID: PMC6996528.
- Solis AG, Klapholz M, Zhao J, Levy M. The bidirectional nature of microbiome-epithelial cell interactions. Curr Opin Microbiol. 2020 Aug;56:45-51. doi: 10.1016/j.mib.2020.06.007. Epub 2020 Jul 9. PMID: 32653776; PMCID: PMC7744412.
- Shi N, Li N, Duan X, Niu H. Interaction between the gut microbiome and mucosal immune system. Mil Med Res. 2017 Apr 27;4:14. doi: 10.1186/s40779-017-0122-9. PMID: 28465831; PMCID: PMC5408367.
- Okumura R, Takeda K. Roles of intestinal epithelial cells in the maintenance of gut homeostasis. Exp Mol Med. 2017 May 26;49(5):e338. doi: 10.1038/emm.2017.20. PMID: 28546564; PMCID: PMC5454438.
- Ghosh S, Whitley CS, Haribabu B, Jala VR. Regulation of Intestinal Barrier Function by Microbial Metabolites. Cell Mol Gastroenterol Hepatol. 2021;11(5):1463-1482. doi: 10.1016/j.jcmgh.2021.02.007. Epub 2021 Feb 18. PMID: 33610769; PMCID: PMC8025057.
- Tarbell JM, Cancel LM. The glycocalyx and its significance in human medicine. J Intern Med. 2016 Jul;280(1):97-113. doi: 10.1111/joim.12465. Epub 2016 Jan 8. PMID: 26749537.
- Wiertsema SP, van Bergenhenegouwen J, Garssen J, Knippels LM. The interplay between the gut microbiome and the immune system in the context of infectious diseases throughout life and the role of nutrition in Optimizing Treatment Strategies. Nutrients. 2021;13(3):886.
- Chabbi-Achengli Y, Coman T, Collet C, Callebert J, Corcelli M, Lin H, et al. Serotonin is involved in autoimmune arthritis through th17 immunity and Bone Resorption. The American Journal of Pathology. 2016;186(4):927–37.












